Using integrated CO2 capture and utilisation concept to produce methane is one solution to recycle CO2. The advantage of this process is that the produced methane has a mature market and infrastructure. However, the product value is low, in particular, compared to H2. Even, if renewable H2 is used, the economics might not be feasible.
From the perspective of research, 2D-layered Ni–MgO–Al2O3 nanosheets were prepared for ICCU and used at temperatures around 250 °C (https://doi.org/10.1002/cssc.201902828). The authors claimed almost 100% CO2 capture efficiency and 100% conversion of captured CO2. However, the presented results are not quantified and the reaction time is very short (~20 s). The high conversion of CO2 might be related to the low CO2 capture capacity in this work (~1.42wt.%). This work provides an important message. By shortening the time of CO2 capture, 100% conversion of the captured CO2 could be achieved. There is a balance between the capacity of CO2 capture and the conversion of the captured CO2.
A relatively excellent ICCU-methanation performance was reported with 3.24 mmol/g CO2 capture capacity and 2.21 mmol/g CH4 formation, using NaNO3/MgO sorbents mixed with Ru/Al2O3 catalysts (https://doi.org/10.1016/j.cej.2021.130369). MgO normally has a low practical CO2 capture capacity. In this work, mesoporous MgO was prepared and also doped with NaNO3. However, excessive sintering of MgO was observed and responsible for the reduced capacity of CO2 capture when the number of sorption cycles increased. The potential evaporation or redistribution of the NaNO3 promoter might also contribute to the decreased CO2 capture capacity. Around 60% of the captured CO2 was converted to methane in this work. However, the use of Ru-based catalysts for ICCU might not be feasible, as ICCU materials need to be used directly in flue gas, where the pollutants could easily deactivate the Ru-based catalysts. It is noted that interesting kinetic studies were reported in this work. This is rare in the current ICCU literature.
MgO was also used as the adsorbent for ICCU-methanation, and Ru/CeO2 with different morphologies acted as the catalyst. Only 0.3 mmol/g CH4 production was reported. The conversion of captured CO2 is around 60%, which is consistent with the above paper. It is noted that cube-CeO2 demonstrated very poor conversion of the capture CO2 (around 2.7%). Interestingly, over 95% CO2 conversion was obtained after 5 cycles of ICCU.

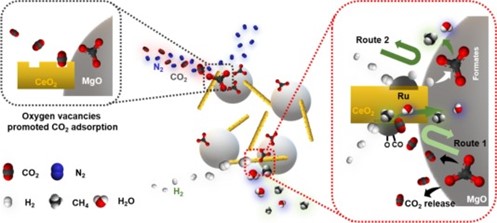
The authors provide useful suggestions for reaction mechanisms and enhancing ICCU-methanation performance as below:
“In situ DRIFTS study indicates that ICCM over Ru/rod-CeO2-MgO proceeds via the formates and CO2 dissociation (Ru-CO species) pathways. The formates pathway do not require gas phase CO2 to release from carbonates. Therefore, it could contribute to achieving ideal ICCM performance (100% CO2 conversion and 100% CH4 selectivity). Improving the formates pathway and inhibiting gas-phase CO2 release in the methanation step could be a promising strategy to achieve perfect ICCM.”
