There is extensive research on carbon capture using adsorption. The reaction mechanism for CO2 capture could be complicated when catalytic sites (e.g. Ni) are involved. Furthermore, the utilisation of captured CO2 is different from conventional gas-gas phase reactions for CO2 conversion. In ICCU, gas-solid reactions are unknown. Should CO2 be released from the sorbent first, then proceed to CO2 reduction, or could the reduction agent (e.g. H2) react directly with the solid phase CO2 adsorbent?
The following sentences are copied from this reference (https://doi.org/10.1016/j.ccst.2022.100052) regarding the reaction mechanism of Ni-based DFMs for ICCU-RWGS.
“Since CH4, CO, and CO2 coexisted in the methanation off-gas, the CO2 conversion was proposed to be a two-step process, namely, the release of CO2 from CaCO3 and the reaction with H2 to produce CH4 and CO (Ma et al., 2021). By comparing the CaO, 1wt%Ni/CaO, and 10wt%Ni/CaO in TPSR tests, it was found that CO was generated on carbonated CaO even though no Ni was added. With increasing the Ni from 1 to 10 wt%, the CH4 yield significantly decreased while CO production was comparable. It was thus inferred that the methanation reaction happened on the Ni site, while the RWGS mainly happened on CaO with the generation of CO (Ma et al., 2021). The generation of CO rather than CH4 was also reported by Sun et al. when using Ce-CaO (without addition of Ni) in the carbonation-methanation cycle (Sun, H. et al., 2022; Sun, S. et al., 2021a). On Ni/CeO2-CaO surface, it was found formate, calcite carbonate, bicarbonate species, and CO species presented after CO2 capture with the in-situ DRIFTS study. After introducing H2, the intensity of formate and calcite carbonate remained unchanged, while that of CO decreased. This indicates that the CO is possibly the main intermediate being converted to CH4 (Sun, H. et al., 2022), as illustrated in Fig. 12. By contrast, the surface formate and carbonate species intensity decreased upon H2 injection with the generation of gas-phase CH4 on Ni-MgO-Al2O3 (Zhou et al., 2020). The authors found that CO is less likely to be the intermediate for methanation as no signal of CO was detected during the FTIR test, indicating that methanation should proceed with direct hydrogenation of formate. Additionally, the carbonate is more likely to bond with metal oxides, it was assumed that the hydrogenation was accompanied by the migration of CO2 to the metallic Ni surface (Zhou et al., 2020), namely the spillover of CO2 as reported by Farrauto et al. (Arellano-Treviño et al., 2019b).”
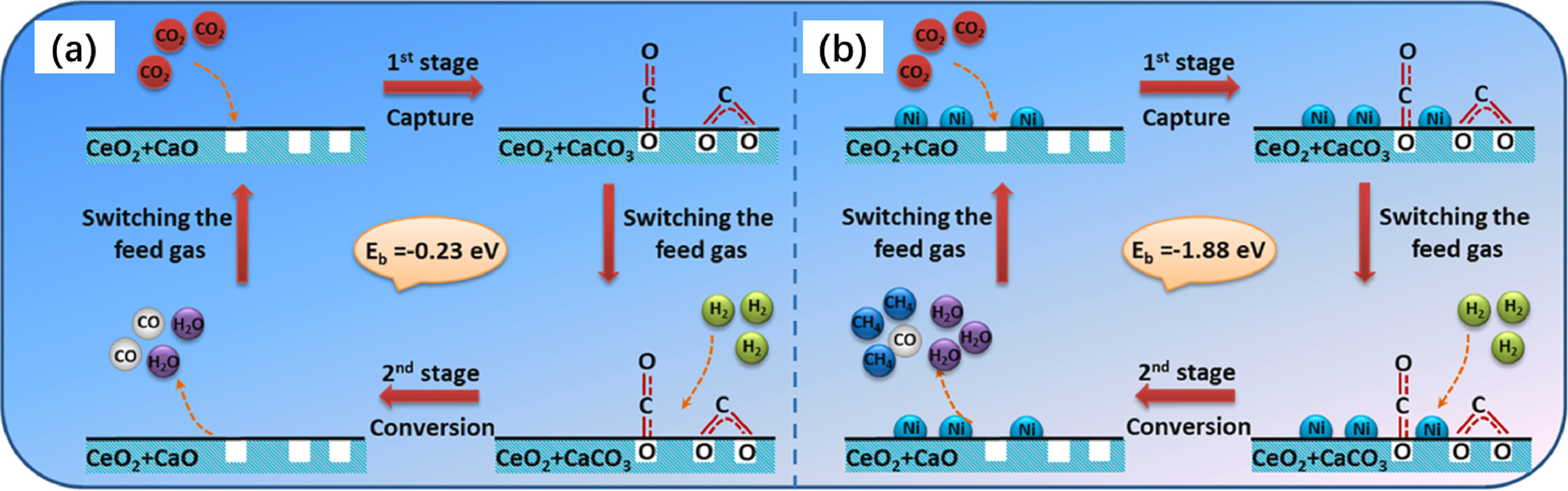
“Fig. 12. Reaction mechanism on (a) CeO2-CaO (b) Ni/CeO2-CaO for CO2 capture and methanation (Sun, H. et al., 2022).”
